Tofacitinib citrate yog ib qho tshuaj kho mob (lub npe lag luam Xeljanz) tsim los ntawm Pfizer rau chav kawm ntawm qhov ncauj Janus kinase (JAK) inhibitors. Nws muaj peev xwm xaiv inhibit JAK kinase, thaiv JAK / STAT txoj hauv kev, thiab yog li inhibit cell signal transduction thiab Related gene expression and activation, siv los kho mob rheumatoid mob caj dab, psoriatic mob caj dab, ulcerative colitis thiab lwm yam kab mob tiv thaiv kab mob.
Cov tshuaj suav nrog peb hom tshuaj: ntsiav tshuaj, cov ntsiav tshuaj tso tawm thiab cov tshuaj hauv qhov ncauj. Nws cov ntsiav tshuaj tau pom zoo thawj zaug los ntawm FDA hauv 2012, thiab daim ntawv tso cai tso tawm tau pom zoo los ntawm FDA thaum Lub Ob Hlis 2016. Nws yog thawj zaug los kho cov pob qij txha rheumatoid. Yan yog JAK inhibitor noj ib hnub ib zaug. Thaum Lub Kaum Ob Hlis 2019, ib qho kev qhia tshiab rau cov tshuaj tiv thaiv-tso tawm tau pom zoo dua rau kev mob hnyav rau mob hnyav rau mob plab (UC). Tsis tas li ntawd, tam sim no theem 3 kev sim tshuaj rau cov quav hniav psoriasis tau ua tiav, thiab lwm qhov rau theem 3 kev kuaj mob tau ua tiav, cuam tshuam nrog kev mob caj dab psoriatic, cov menyuam yaus idiopathic mob caj dab, thiab lwm yam kev qhia. Qhov zoo ntawm cov ntsiav tshuaj sustained-tso tawm uas ua haujlwm ntev thiab tsuas yog yuav tsum tau noj ib zaug ib hnub yog qhov tsim nyog rau kev tswj hwm thiab tswj cov neeg mob cov kab mob.
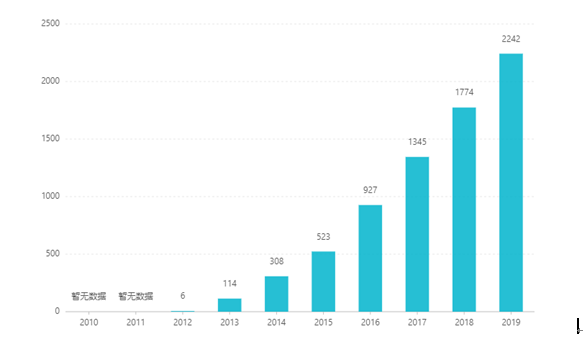
Txij li thaum nws cov npe, nws qhov muag tau nce xyoo dhau los, nce mus txog US $ 2.242 billion hauv 2019. Hauv Suav teb, daim ntawv tshuaj ntsiav tshuaj tau pom zoo rau kev lag luam hauv lub Peb Hlis 2017, thiab nkag mus rau hauv kev tuav pov hwm kho mob qeb B catalog los ntawm kev sib tham hauv 2019. Qhov tseeb yeej bid yog RMB 26.79. Txawm li cas los xij, vim muaj kev cuam tshuam txog kev ua haujlwm siab ntawm kev npaj-tso tawm, daim ntawv tshuaj no tseem tsis tau muaj muag hauv Suav teb.
JAK kinase plays lub luag haujlwm tseem ceeb hauv kev mob, thiab nws cov inhibitors tau pom tias kho qee yam kab mob autoimmune. Txog tam sim no, 7 JAK inhibitors tau pom zoo thoob ntiaj teb, suav nrog Leo Pharma's Delgocitinib, Celgene's Fedratinib, AbbVie's upatinib, Astellas's Pefitinib, Eli Lilly's Baritinib Thiab Novartis's Rocotinib. Txawm li cas los xij, tsuas yog tofacitinib, baritinib thiab rocotinib tau pom zoo hauv Suav teb ntawm cov tshuaj hais saum toj no. Peb tos ntsoov rau Qilu's "Tofatib Citrate Sustained Release Tablets" tau pom zoo sai li sai tau thiab muaj txiaj ntsig zoo rau cov neeg mob ntau dua.
Hauv Suav teb, thawj qhov kev tshawb fawb tofacitib citrate tau pom zoo los ntawm NMPA thaum Lub Peb Hlis 2017 rau kev kho mob ntawm cov neeg laus RA cov neeg mob uas tsis muaj txiaj ntsig lossis tsis kam rau methotrexate, raws li lub npe lag luam Shangjie. Raws li cov ntaub ntawv los ntawm Meinenet, kev muag khoom ntawm tofacitib citrate ntsiav tshuaj hauv Tuam Tshoj cov chaw kho mob pej xeem hauv xyoo 2018 yog 8.34 lab yuan, uas qis dua nws qhov muag thoob ntiaj teb. Ib feem loj ntawm qhov laj thawj yog tus nqi. Nws tau tshaj tawm tias Shangjie tus nqi muag khoom thawj zaug yog 2085 yuan (5mg * 28 ntsiav tshuaj), thiab tus nqi txhua hli yog 4170 yuan, uas tsis yog lub nra me me rau tsev neeg zoo tib yam.
Txawm li cas los xij, nws tsim nyog ua kev zoo siab tias tofacitib tau suav nrog hauv 2019 "National Basic Medical Insurance, Work Injury Insurance and Maternity Insurance Drug List" los ntawm National Medical Insurance Administration tom qab sib tham thaum lub Kaum Ib Hlis 2019. Nws tau tshaj tawm tias tus nqi them txhua hli yuav raug txo. mus rau hauv qab 2,000 yuan tom qab tus nqi txiav yog sib tham, uas yuav zoo heev txhim kho qhov muaj ntawm tshuaj.
Thaum Lub Yim Hli 2018, Patent Reexamination Board of State Intellectual Property Office tau ua qhov kev txiav txim siab tsis pub dhau 36902 thov rau kev siv tsis raug, thiab tshaj tawm tias qhov tseem ceeb patent ntawm Pfizertofatib, cov ntaub ntawv sib xyaw patent, vim qhov tsis txaus nthuav tawm ntawm cov lus qhia tshwj xeeb. Txawm li cas los xij, patent ntawm Pfizertofatiib siv lead ua daim ntawv (ZL02823587.8, CN1325498C, hnub thov 2002.11.25) yuav tas rau xyoo 2022.
Lub Insight database qhia tau hais tias, ntxiv rau cov kev tshawb fawb thawj zaug, tsib cov tshuaj generic ntawm Chia Tai Tianqing, Qilu, Kelun, Yangtze River, thiab Nanjing Chia Tai Tianqing tau pom zoo rau kev lag luam hauv domestic tofacitinib ntsiav tshuaj formulations. Txawm li cas los xij, rau hom ntsiav tshuaj uas tau tso tawm, tsuas yog thawj qhov kev tshawb fawb Pfizer tau xa daim ntawv thov kev lag luam rau lub Tsib Hlis 26. Qilu yog thawj lub tuam txhab hauv tsev xa daim ntawv thov kev lag luam rau cov qauv no. Tsis tas li ntawd, CSPC Ouyi yog nyob rau theem BE mus sib hais.
Changzhou Pharmaceutical Factory (CPF) yog lub chaw tsim tshuaj paus ntawm APIs, tiav formulations nyob rau hauv Tuam Tshoj, uas nyob rau hauv Changzhou, Jiangsu xeev. CPF tau tsim muaj nyob rau hauv 1949. Peb tau mob siab rau hauv Tofacitinib Citrate los ntawm 2013, thiab xa DMF lawm. Peb tau sau npe hauv ntau lub teb chaws, thiab tuaj yeem pab txhawb koj nrog cov ntaub ntawv zoo tshaj plaws txhawb nqa rau Tofacitinib Citrate.
Post lub sij hawm: Lub Xya hli ntuj-23-2021