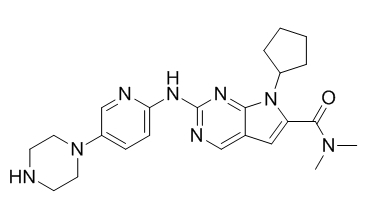
Ribociclib 1374639-75-4
Kev piav qhia
Ribociclib (LEE01) yog ib qho tshwj xeeb CDK4/6 inhibitor nrog IC50 qhov tseem ceeb ntawm 10 nM thiab 39 nM, raws li, thiab muaj ntau dua 1,000-fold tsis muaj zog tiv thaiv cyclin B / CDK1 complex.
Hauv Vitro
Kev kho lub vaj huam sib luag ntawm 17 neuroblastoma kab ntawm tes nrog Ribociclib (LEE011) hla plaub-log koob ntau (10 txog 10,000 nM).Kev kho mob nrog Ribociclib ho inhibits substrate adherent kev loj hlob txheeb ze rau kev tswj nyob rau hauv 12 ntawm 17 neuroblastoma cell kab kuaj (txhais tau tias IC50 = 306±68 nM, xav txog cov kab rhiab nkaus xwb, qhov rhiab heev txhais tau tias yog IC50 tsawg dua 1μM. Ribociclib kev kho mob ntawm ob kab mob neuroblastoma ntawm tes (BE2C thiab IMR5) nrog kev ua kom pom tseeb ntawm CDK4 / 6 inhibition ua rau muaj kev cuam tshuam ntawm cov hlwb hauv G0 / G1 theem ntawm lub voj voog ntawm tes.Qhov kev ntes G0 / G1 no dhau los ua qhov tseem ceeb ntawm Ribociclib concentrations ntawm 100 nM (p = 0.007) thiab 250 nM (p = 0.01), feem.
CB17 immunodeficient nas bearing BE2C, NB-1643 (MYCN amplified, rhiab hauv vitro), lossis EBC1 (non-amplified, resistant in vitro) xenografts raug kho ib hnub ib hnub rau 21 hnub nrog Ribociclib (LEE011; 200 mg / kg) lossis nrog rau tswj tsheb.Qhov kev siv tshuaj no tau txais txiaj ntsig zoo, vim tias tsis muaj qhov hnyav lossis lwm yam cim ntawm toxicity tau pom nyob rau hauv ib qho ntawm cov qauv xenograft.Cov qog loj hlob qeeb qeeb thoob plaws hauv 21 hnub ntawm kev kho mob hauv nas harboring BE2C lossis 1643 xenografts (ob leeg, p<0.0001), txawm hais tias kev loj hlob rov qab kho tom qab kho.
Cia
| Hmoov | -20 ° C | 3 xyoo |
| 4 ° C | 2 xyoo | |
| Hauv cov kuab tshuaj | -80 ° C | 6 hli |
| -20 ° C | 1 hli |
Cov qauv tshuaj





Tswv yim18Quality Consistency Evaluation tej yaam num uas tau pom zoo4, thiab6tej yaam num raug tso cai.

Advanced thoob ntiaj teb kev tswj hwm qhov system tau tso lub hauv paus ruaj khov rau kev muag khoom.

Kev saib xyuas zoo ua haujlwm los ntawm tag nrho lub neej voj voog ntawm cov khoom kom ntseeg tau tias qhov zoo thiab kho cov nyhuv.

Pab Pawg Kev Tswj Xyuas Kev Tswj Xyuas Kev Ua Haujlwm txhawb nqa qhov kev xav tau zoo thaum lub sijhawm thov thiab tso npe.


Kauslim Countec Lub raj mis ntim kab


Taiwan CVC Lub raj mis ntim kab


Ltalis CAM Board Ntim Kab

German Fette Compacting Tshuab

Nyiv Viswill Tablet Detector

DCS Control Room










